En
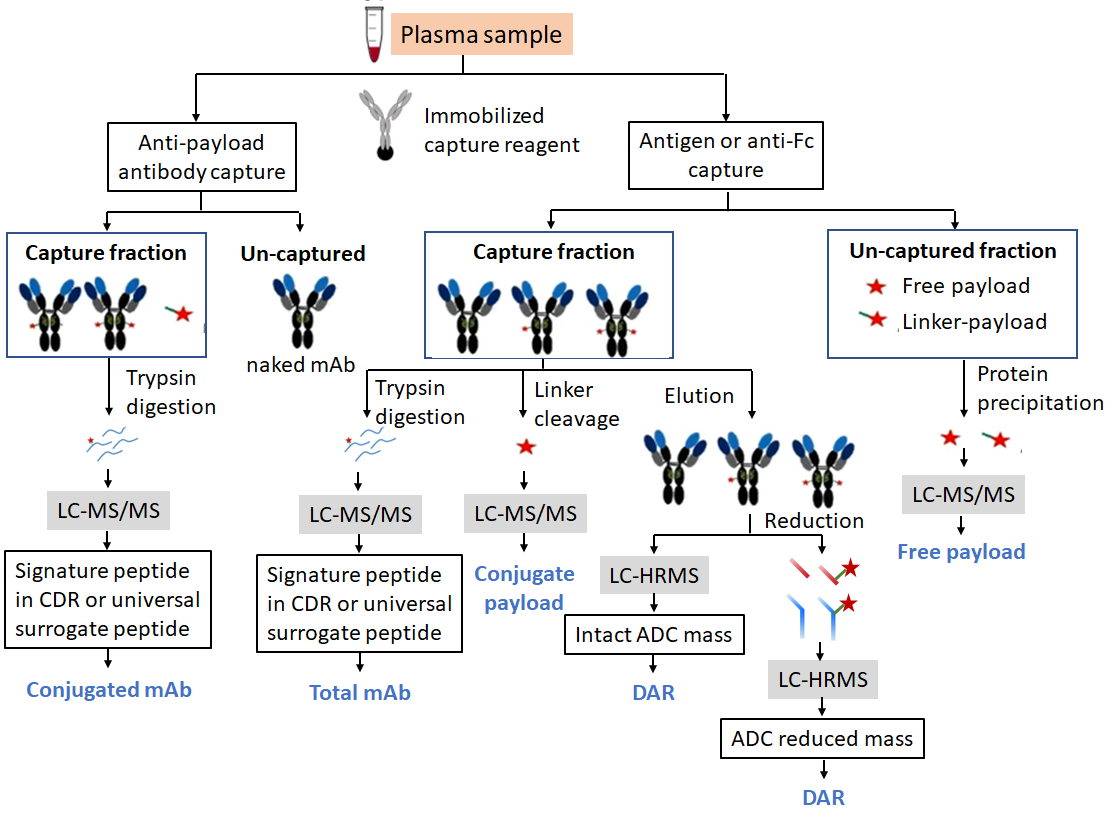
Bioanalysis of ADC drugs plays an important role in pharmacokinetic and toxicity evaluation. Due to the complexity of ADC drug structures, qualitative and quantitative analyses are often required for several of their major forms of existence, including drug-antibody ratio (DAR) total antibodies, free payload, and conjugated antibodies.
For the detection of DAR, Total mAb and Conjugated mAb of ADC drugs, this is generally done by affinity capture. A biotinylated antigen or an anti-payload antibody is used to bind to the ADC, which is then captured by affinity-primed magnetic beads. After eluting it from the magnetic beads, it can be reduced and then loading to LC-HRMS to obtain DAR, or it can be digested with trypsin and detected at the peptide level to obtain information such as Total mAb and Conjugated mAb.
Representative mass spectra of conventional cysteine-conjugated ADC for DAR determination from plasma incubation at 0, 72, and 144 h.
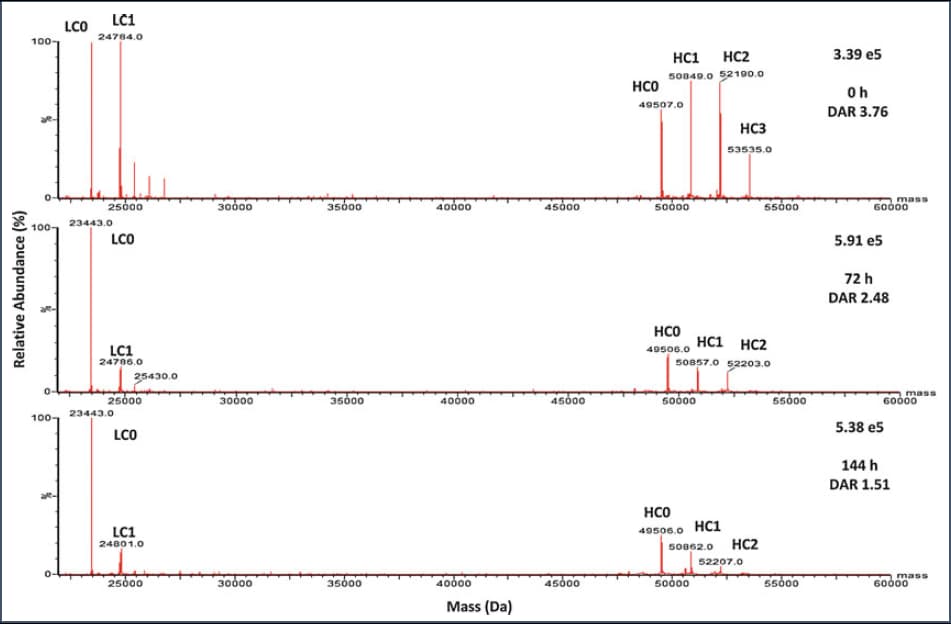
From Wei C. Assessing ADC Plasma Stability by LC-MS Methods. Methods Mol Biol. 2020;2078:353-359.
Call Us
Address
160E Tasman Drive, San Jose, CA 95134