En
Plasma stability testing of ADC drugs is an important preclinical toxicological and pharmacokinetic evaluation step. It aims to determine the integrity of the ADC molecule in the human plasma environment and the stability of the drug-antibody conjugate to avoid systemic toxicity and adverse reactions due to premature release of the load. This process typically exposes ADCs to plasma for a specific period of time to monitor structural changes, including the drug-antibody ratio, release of the drug payload, and degradation of the antibody molecule. Through plasma stability testing, researchers are able to assess the stability of ADC drugs in the circulation, the duration of drug effect, and the risk of potential adverse reactions to ensure their safety and efficacy.
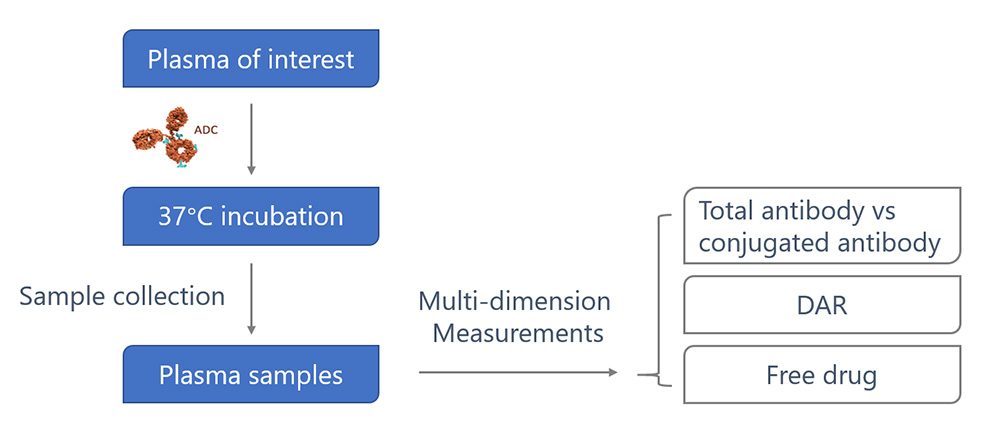
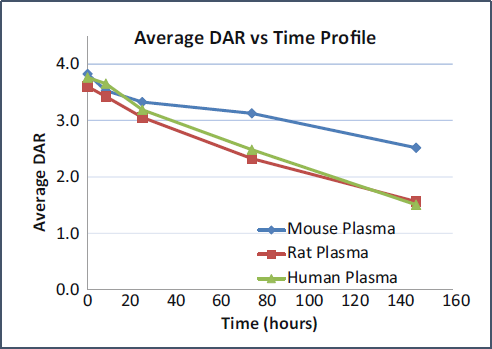
Average DAR profiles of a conventional cysteine-conjugated ADC Trastuzumab-maleimido-caproylvaline-citruline-p-amino-benzyloxy-carbonyl-payload from the in vitro plasma incubation.
Call Us
Address
160E Tasman Drive, San Jose, CA 95134