En
Oligonucleotides are a class of short-chain nucleic acids composed of deoxyribonucleotides or ribonucleotides. They can form base pairs with DNA, mRNA, or pre-mRNA through the principle of complementary base pairing. By precisely "silencing" certain genes, oligonucleotides can inhibit the expression of many proteins, thereby achieving the goal of curing diseases. This oligonucleotide analysis process is fundamental for elucidating genetic information, understanding gene expression patterns, and developing novel therapeutic strategies in areas such as genomics, genetics, and drug discovery.
INOMIXO can provide quality research and quality control for oligonucleotide drugs such as ASOs and siRNAs, with CMA-ISO9001 quality certification, and a quality system that meets the requirements of NMPA and FDA.
Quality Attribute | Analytes | Attribute |
Characterization | Reference batch of DS | MW/ Sequence/ Tm/Total content/Absorbance coefficient |
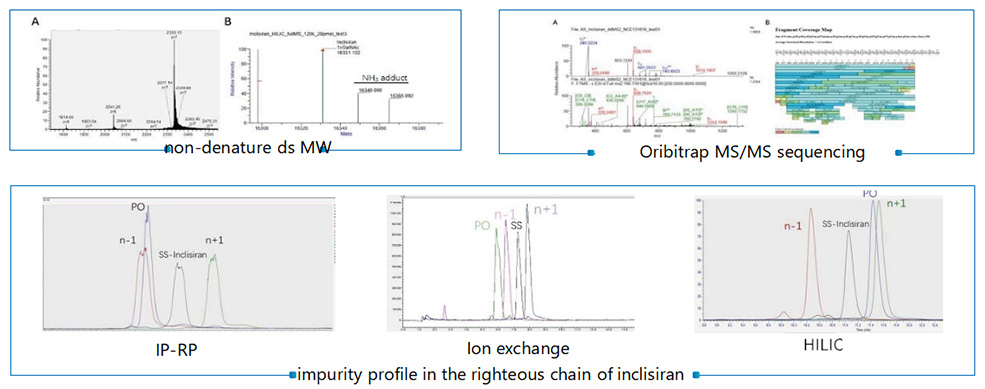
Identification | Release batch of DS | MW/ Tm/ MS-MS sequencing |
Release batch of DP | MW | |
Assay | Release batch of DS and DP | Total content/Purity or assay |
Purity and impurity profile | Release batch of DS and DP | Chromatographic purity (IC-RP) |
Chromatographic purity (IP-RP) | ||
Chromatographic purity (HILIC) | ||
CE purity | ||
Impurities | Release batch of DS | Residual solvents/ Heavy metal residues/ Process-related impurities |
Counterion testing | Release batch of DS | |
Water content | Release batch of DS | |
Safety | Release batch of DS and DP | Endotoxin |
Bioburden |
Please note that the table provided only includes part of test items. For more detailed information on each project, please download the support documents or contact info@inomixo.com.
Call Us
Address
160E Tasman Drive, San Jose, CA 95134