En
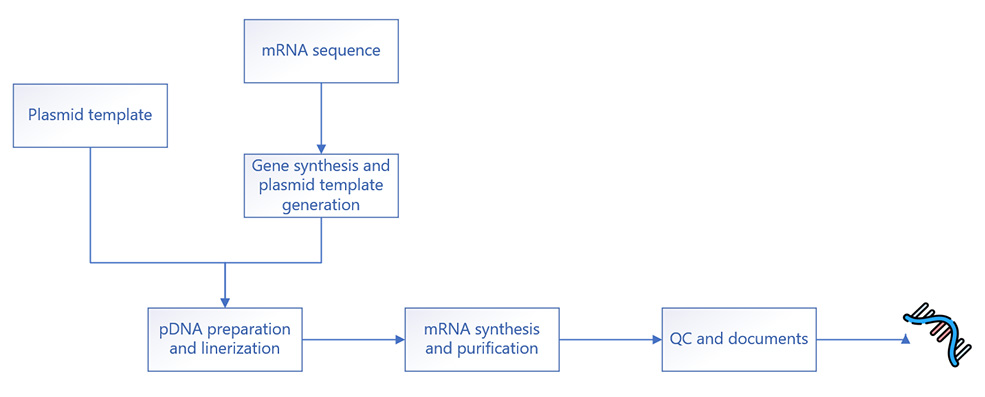
As mRNA programs progress, the demand for both quantity and quality escalates. At INOMIXO, we specialize in producing high-quality mRNA tailored to your specifications, utilizing scalable processes capable of delivering from milligram to multi-gram quantities. Moreover, we offer linearized plasmid DNA to support your in vitro transcribed mRNA synthesis requirements. With our commitment to excellence, we ensure that your mRNA needs are met efficiently and reliably, empowering the advancement of mRNA-based therapies.
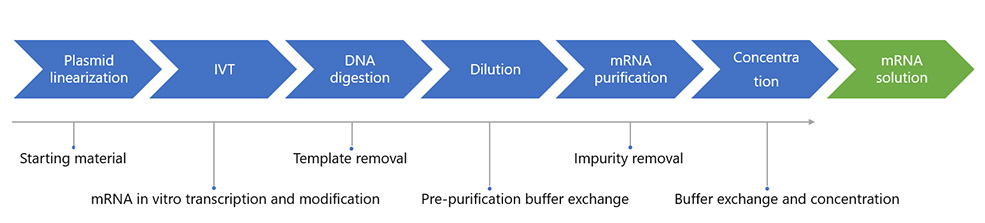
We can offer enzymatic capping (two-step method) and co-transcriptional capping (one-step method) to add caps at the 5' end of RNA, enhancing RNA stability and translational efficiency. We also analyze and refine the composition of nucleotides, reagents, and enzymes, as well as optimize process conditions in the development phase.
At INOMIXO, we provide comprehensive testing for critical attributes such as purity, identity, concentration, total residual protein content, residual DNA template, residual endotoxin content and bioburden etc.
Quality | Attribute | Method |
Identity | Sequence Confirmation | Sanger Sequencing |
Content | RNA Concentration | UV |
Potency | Activity Assay | In vitro Transcription Assay |
Purity/ Integrity | 5’ capping efficiency | LC-MS/RP-HPLC |
3’ poly(A) (% or length) | LC-MS/RP-HPLC | |
A260/A280 | UV | |
Impurity | Product Related impurities – dsRNA | ELISA/dot-blot |
Process Related impurities - Residual Protein | Fluorescent Dye Method | |
Process Related impurities - Residual Template | qPCR | |
NTPs | HPLC | |
Safety | Endotoxin | CHP<1143> |
Bioburden | CHP<1101> |
Go to the next platform phase:
Call Us
Address
160E Tasman Drive, San Jose, CA 95134