En
The custom manufacturing of mRNA-LNP drug products involves the specialized production of mRNA therapeutics encapsulated within lipid nanoparticles. This process encompasses the precise formulation, assembly, and quality control of lipid nanoparticles loaded with mRNA molecules designed for specific therapeutic purposes.
INOMIXO is dedicated to providing Phase I R&D and pilot services from RNA molecular design and optimization to drug CMC development and production and IND filing. As a company specializing in mRNA manufacturing, we offer tailored solutions leveraging a variety of tools to optimize your mRNA program to meet your project needs.
Plasmid development and production
mRNA process development
LNP formulation development
Quality control analysis services
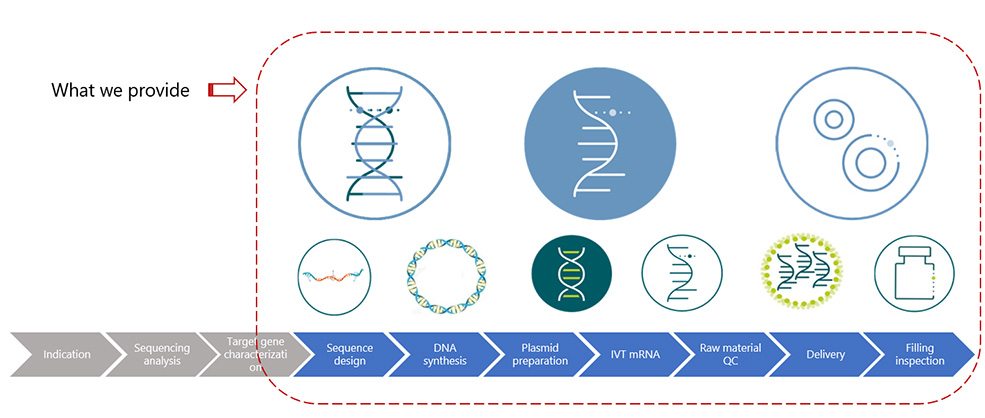
| Drug Substance Production | Drug Product Production |
| Our drug substance production service ensures the consistent and efficient production of high-quality API, meeting the requirements for drug development, clinical trials, and commercialization. | Our drug product production service ensures the efficient and consistent production of high-quality pharmaceutical products, tailored to meet the specific needs of our clients and regulatory requirements. |
| Learn More | Learn More |
Call Us
Address
160E Tasman Drive, San Jose, CA 95134