En
Our Drug Product Production process involves formulating, manufacturing, and packaging pharmaceutical products for distribution and use. We focus on ensuring the safety, efficacy, and quality of our products through meticulous attention to detail and adherence to rigorous standards. Utilizing advanced technologies and experienced personnel, we aim to deliver products that meet regulatory requirements and satisfy customer needs. Our commitment to efficiency, reliability, and patient safety drives every aspect of our production processes.
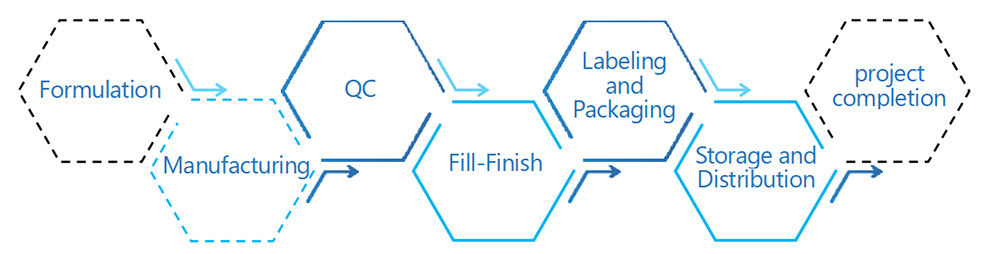
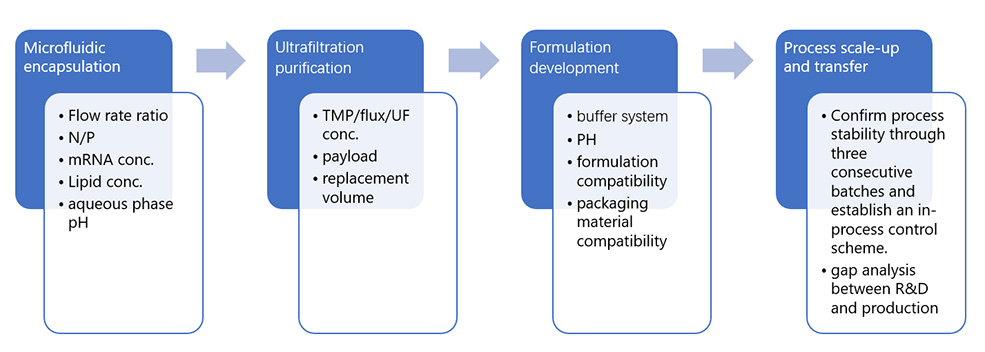
Developing an appropriate formulation, including selecting suitable carriers and additives, to ensure the stability and proper delivery performance of mRNA manufacturing drug products.
At INOMIXO, we provide rigorous quality control and testing at each stage of the production process to ensure that the products meet predefined specifications and standards. This includes inspection of raw materials, intermediate products, and final products.
Quality | Attribute | Method |
Identity | Sequence Confirmation | Sanger Sequencing |
Poly length | LC-MS | |
Lipid identification | HPLC-CAD | |
Content | Encapsulation Efficiency(EE%) | RiboGreen |
Sucrose content | UPLC-CAD | |
Lipid content | HPLC-CAD | |
Integrity | mRNA integrity | CE |
LNP size and PDI | DLS | |
Impurity | Lipid impurities | HPLC-CAD |
mRNA fragments | IP-RP-HPLC | |
Potency | Activity Assay | Cell Based Assay |
Safety | Endotoxin | CHP<1143> |
Bioburden | CHP<1101> |
Call Us
Address
160E Tasman Drive, San Jose, CA 95134